Advanced Example 4: Post-Hoc Tests for Repeated Measures
Post-hoc comparisons in designs that include within-subject (repeated measures) effects require constructing appropriate error terms for the comparisons (for details, see Post-hoc tests for repeated measures effects in Comparison with other General Linear Model Programs). Post-hoc comparisons tests in such designs can be illustrated using the example data file Heart2.sta. These data are presented in Milliken and Johnson (1992, p. 332). A different Drug was given to three groups of subjects. Subjects' heart rates were measured at four different times, thus, Time represents a within-subject factor at four levels. Milliken and Johnson (1992) analyzed this data using a 3 (between) by 4 (within) factorial design. Thus, both between and within error terms are available for testing hypotheses about differences in heart rates.
- Specifying the Design
- Open the Heart2.sta data file and start the General Linear Models (GLM) module:
Ribbon bar. Select the Home tab. In the File group, click the Open arrow and select Open Examples to display the Open a STATISTICA Data File dialog box. The Heart2.sta data file is located in the Datasets folder. Then, select the Statistics tab. In the Advanced/Multivariate group, click Advanced Models and from the menu, select General Linear to display the General Linear Models (GLM) Startup Panel.
Classic menus. On the File menu, select Open Examples to display the Open a STATISTICA Data File dialog box. The Heart2.sta data file is located in the Datasets folder. Next, from the Statistics - Advanced Linear/Nonlinear Models submenu, select General Linear Models to display the General Linear Models (GLM) Startup Panel.
Select General linear models as the Type of analysis and Quick specs dialog as the Specification Method. Then click the OK button to display the GLM General linear models Quick Specs dialog box.
Click the Variables button to display a standard variable selection dialog box.
Specify Time1 through Time4 as the Dependent variables and Drug as the Categorical pred. variable. Click the OK button.
On the Quick tab of the GLM General linear models dialog box, click the Within effects button to display the Specify within-subjects factors dialog box. Specify one repeated measure with 4 No. of levels and the Factor Name of R1. Click the OK button to return to the GLM General linear models Quick Specs dialog box, and then click the OK button to display the GLM Results dialog box.
- Results
- On the
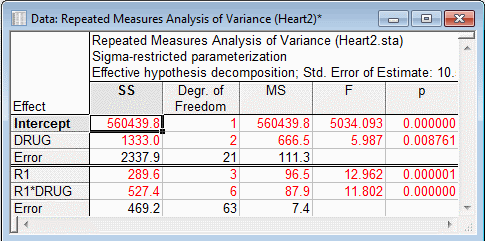
Quick tab, click the All effects button to review the Repeated Measures Analysis of Variance spreadsheet.
Three types of post-hoc comparisons are possible: 1) average heart rate for different drugs at the same time, 2) average heart rate for the same drug at different times, and 3) average heart rate for different drugs at different times. Winer, Brown, and Michels (1991, p. 526-531; see also Milliken and Johnson, 1992, p. 322-350) recommend using the 1) between, 2) within, and 3) pooled error term for these three types of comparisons, respectively. These are the default error terms used for the respective comparisons for between-subject by within-subject interaction effects. The error term for the comparison is reflected in the standard error for the difference of pairs of means that are compared.
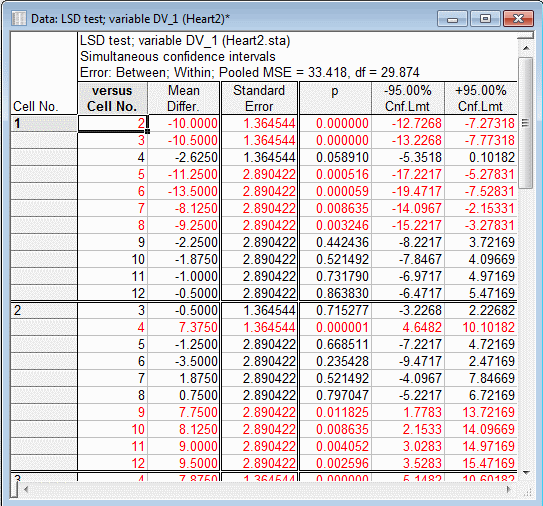
The spreadsheet displayed below shows the comparisons of all means with the means for the first two Drug by Time combinations. (Click the More results button to display the larger Results dialog box. On the Post-hoc tab, select the R1*Drug Effect. In the Display group box, select the Confidence intervals option button, and then click the Fisher LSD button.)
Note: for comparisons for between-subject by within-subject interaction effects, three other types of options are also available. The between error can be used for all comparisons, the within error can be used for all comparisons, or you can specify a custom error term for all comparisons. These options provide great flexibility for performing post-hoc comparisons in designs with within-subject (repeated measures) effects.See also GLM - Index.